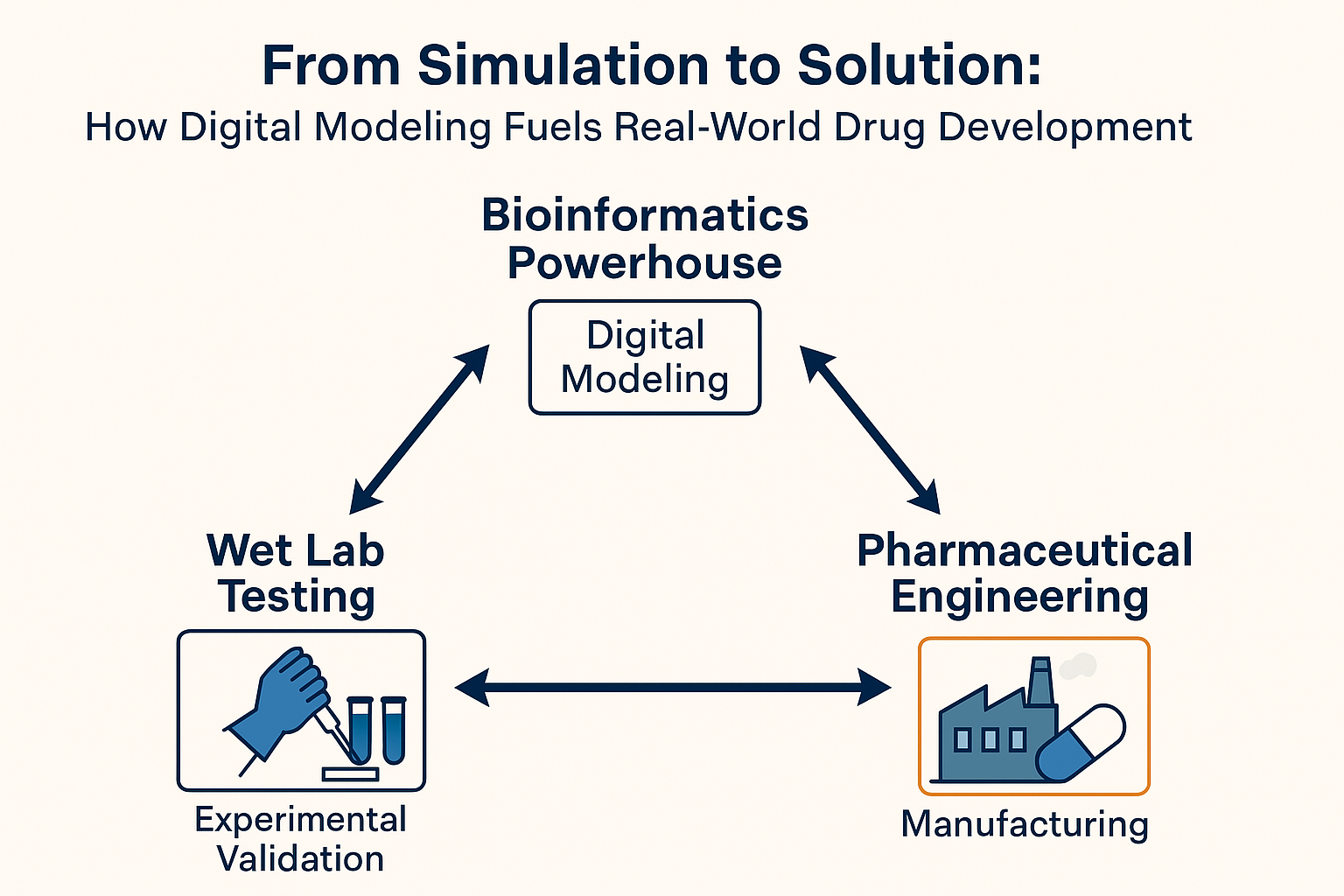
In the current pharmaceutical
environment, the path to innovative therapies starts on a screen rather than on
a production line or petri dish. Digital intelligence is driving medication
research to become more precise, linked, and agile. In silico modeling is at
the center of this change, a digital powerhouse that acts as the engine and
compass for industrial-scale manufacturing and experimental research. These
three domains—pharmaceutical engineering, wet lab testing, and prediction
technologies—create a dynamic feedback loop. Each domain feeds into the others
in an ongoing triangle of innovation.
Digital Modeling: The
Bioinformatics Powerhouse
Bioinformaticians use extensive computational infrastructure to model the behavior of molecular structures before a single molecule is created or tested. Digital modeling makes use of high-performance computers, bioinformatics, and artificial intelligence to:
1. Check for possible biological activity in billions of chemical structures.
2. Predict molecular interactions, including the target proteins' binding affinities.
3. Calculate the absorption, distribution, metabolism, and excretion (ADME) of pharmacokinetics.
4. Anticipate toxicological profiles well in advance of live testing.
By serving as a filter, these simulations reduce enormous compound libraries to a small number of promising candidates. Structure-based drug design, which simulates the interactions of particular chemicals with three-dimensional protein structures, is one of the most revolutionary techniques in this field. Other techniques that create better-informed pipelines include molecular docking, machine learning classifiers, and quantitative structure-activity relationship (QSAR) modeling.
Notably, in silico design
precision has increased thanks to platforms like AlphaFold and Pro3D, which can
now predict protein structures with startling accuracy. The final outcome? Bioinformaticians have the authority to
decide which chemicals should be developed further and which should be
abandoned based on evidence.
Experimental Validation: From
Code to Culture
Once a compound passes the digital inspection, it enters the wet lab. Here, a variety of biological tests are conducted by experimental scientists to evaluate the compound's practicality. These assessments are intended to ascertain:
1. How effectively the substance suppresses or stimulates its biological target.
2. Its effectiveness in animal models, tissue cultures, and cell lines.
3. Adverse responses, toxicological effects, and off-target outcomes.
However, this stage goes beyond simple confirmation. It is also diagnostic, revealing subtleties that the simulations could have overlooked. For instance, a substance may bind effectively in silico but not penetrate cell membranes in vitro. Alternatively, the emergence of unanticipated metabolites in biological systems could complicate the treatment profile.
The important thing is that this
lab data is not isolated. It returns to the digital realm, where it trains
machine learning algorithms, improves computational models, and adjusts
parameters for the subsequent prediction cycle. One of the main advantages of contemporary drug discovery is the
learning system this produces, where each cycle gets better than the one before
it.
Pharmaceutical Engineering:
Scaling the Science
Successful candidates now enter the realm of formulation science and pharmaceutical manufacturing. The aim is to convert encouraging laboratory findings into a pharmaceutical product that is both scalable and shelf-stable. Variables taken into account by formulation engineers include:
1. Pharmacological stability and solubility of the active component.
2. Selection of excipients and delivery systems (e.g., sustained-release capsules, injectables, and tablets).
3. The best possible dosage and bioavailability to optimize the therapeutic outcome.
4. Adherence to regulations for uniformity, safety, and effectiveness.
Modeling is also helpful at this stage. Simulations can forecast the potential effects of formulation modifications on performance. AI is used in advanced manufacturing to streamline production processes, lower waste, increase yield, and guarantee batch consistency.
Within the feedback loop, manufacturing
data enters the lab and computational realms in reverse. If a compound proves
difficult or challenging to scale, it may require reformulation. Moreover, if
degradation occurs during production, it may be necessary to update stability
models. The entire development chain is strengthened by this iterative loop.
Triangular Feedback Loop: A Model
of Interdependence
These three domains don’t operate in silos. Data flows in all directions as they form a triangle. Every triangle corner benefits and enlightens the others:
1. Drug discovery is started with accuracy and foresight thanks to digital modeling.
2. The predictions are verified and improved by wet lab testing.
3. Pharmaceutical engineering provides ideas for optimization and converts findings into treatments.
This cyclical interplay creates a
responsive ecosystem, where each drug's success, and failure, benefits the one
before it. It expedites schedules, lowers expenses, and—above all—provides
patients with more individualized and efficient care.